Is my AI a Medical Device? Qualification for the EU Market.
- kh31091
- 20. Okt. 2023
- 5 Min. Lesezeit
Aktualisiert: 22. Okt. 2023

AI-driven software is increasingly used in medicine, yet determining whether such software qualifies as a medical device can be tricky. Imagine a sophisticated system analyzing MRI scans to pinpoint cancer—clearly a medical device. But what about software that merely sifts through patient data, presenting it without suggesting a treatment path? That's where the waters get murky.
This article delves into the legal intricacies of AI software's medical device classification. We'll reference guidelines from the MDR and the Medical Device Coordination Group (MDCG), provide real-world examples, and offer advice on navigating this classification for your AI product.
The Law: Qualification Guidance in the MDR
Let's cut to the chase: The document that determines whether your AI product is a medical device or not is the Medical Device Regulation (MDR) 2017/745. In particular, see Chapter I, Article 2: the definition of the term "medical device".
Here are the parts relevant for AI-based Software:
(1) ‘medical device’ means [...] software intended by the manufacturer to be used [...] for human beings for one or more of the following specific medical purposes:
diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease,
diagnosis, monitoring, treatment, alleviation of, or compensation for, an injury or disability,
investigation [...] of a physiological or pathological process or state
The majority of our clients develop devices for medical imaging, and very clearly fall into the first one of these bullet points.
However, there is some vagueness in the MDR's definition. One of the more ambiguous elements in the MDR's definition is the phrase "intended for diagnosis". This is a phrase that seems straightforward at first glance, but upon closer examination, raises several questions. How does one define “intent”? And how much involvement in the diagnostic process does a tool need to have before it's classified as a medical device? Consider software that solely displays a radiology image. Without analytical or interpretive features, it's just visualizing data. Does this alone classify it as "intended for diagnosis"? While a doctor might use it for diagnosis, the software isn't actively aiding in the process. But then, where's the boundary? Can it measure specifics, adjust contrast, or allow annotations without being a medical device?
Thankfully, there is more guidance available.
Nearly the Law: MDCG Guidance
The Medical Device Coordination Group (MDCG) is an influential body established by the European Union to support the consistent application and interpretation of the MDR. Their guidance, while not legally binding, is highly regarded and often treated as the de facto standard by industry professionals and regulators alike in Europe.
Here's what they have to say on our topic, in the document MDCG 2019-11:
For example, “Simple search”, which refers to the retrieval of records by matching record metadata against record search criteria or to the retrieval of information does not qualify as medical device software (e.g. library functions).
[...]
Software which is intended to process, analyse, create or modify medical information may be qualified as a medical device software if the creation or modification of that information is governed by a medical intended purpose. For example, the software which alters the representation of data for a medical purpose would qualify as a medical device software. (e.g. “searching image for findings that support a clinical hypothesis as to the diagnosis or evolution of therapy” or “software which locally amplifies the contrast of the finding on an image display so that it serves as a decision support or suggests an action to be taken by the user”).
[...]
However, altering the representation of data for embellishment/cosmetic or compatibility purposes does not readily qualify the software as medical device software.
Unfortunately, there is no clear-cut point that distinguishes e.g. between embellishing and modifying medical information, leaving a large area of uncertainty. Nevertheless, this text provides an initial idea of what types of software are classified as medical devices. The next chapter in the document goes into a bit more detail.
Decision Steps to assist qualification of MDSW
The most useful guidance regarding AI qualification is found in chapter 3.3 of MDCG 2019-11. It comes in form of a decision tree, which we've embellished:
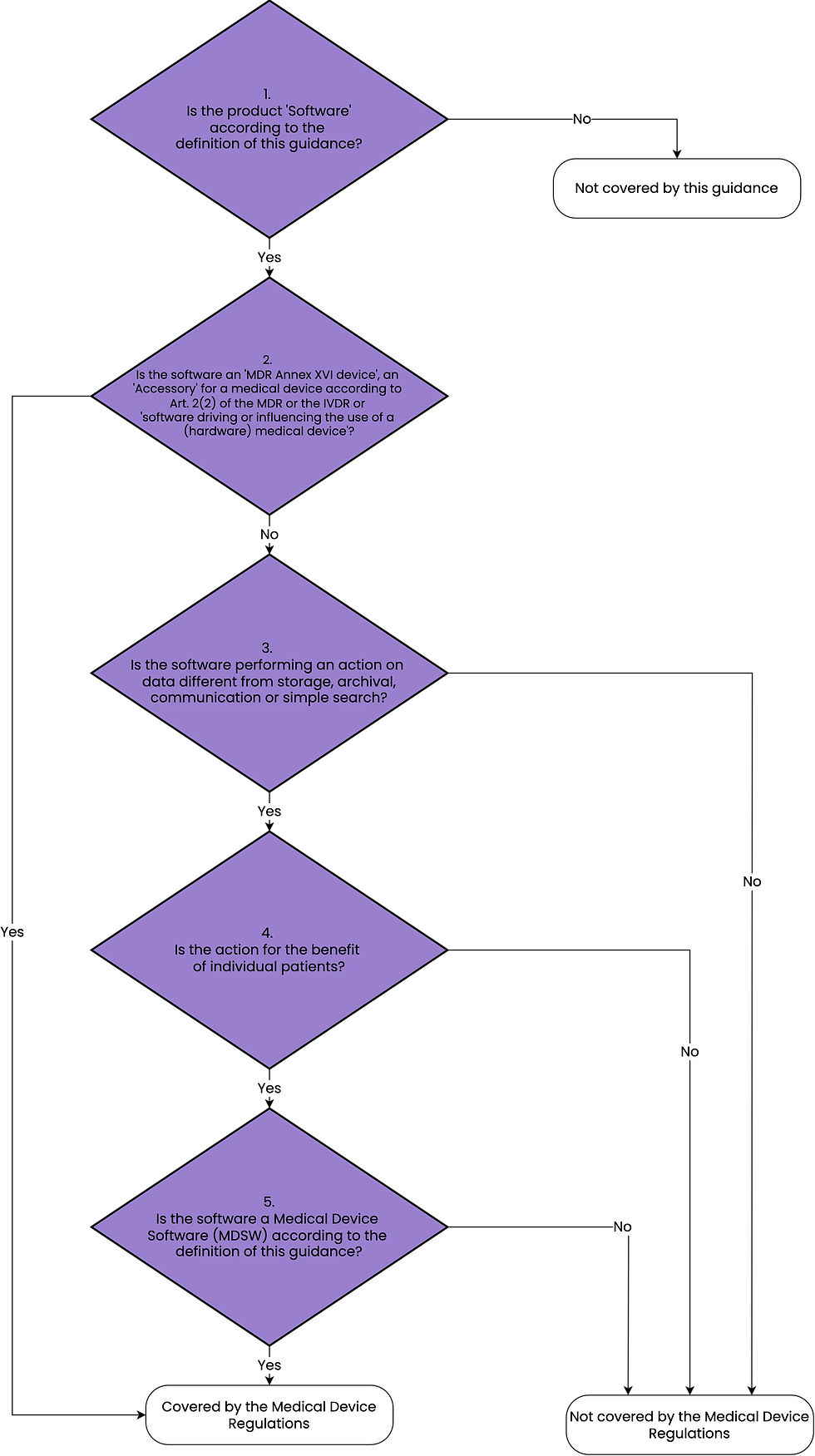
In particular, decision steps 3 and 4 are relevant to AI software.
Decision step 3 further clarifies the difference between data processing techniques. It includes storage, archiving, and communication in the list of activities that do not necessarily make software a medical device. This provides a definitive solution to the previous confusion: The definition in the MDR suggests that displaying any kind of diagnosis-relevant information may automatically make my product a medical device. However, this is not the case - trivial information processing such as storage, archiving, communication, simple search, or embellishment that delivers diagnosis-relevant information does not automatically make AI a medical device.
What about the other question we had earlier? What about cases in which the product does not directly provide a diagnosis, but may have medical implications, such as a population-based study using machine learning?
Let's look at decision step 4. It is described in more detail:
Decision step 4: is the action for the benefit of individual patients?
Examples of software which are not considered as being for the benefit of individual patients are those which are intended only to aggregate population data, provide generic diagnostic or treatment pathways (not directed to individual patients), scientific literature, medical atlases, models and templates as well as software intended only for epidemiological studies or registers.
We've got our answer: No, not every AI that could have medical implications is necessarily a medical device.
However, it should be noted that there is a wide grey area in this regard. For example, if an AI system is used to search for and compile literature on a specific disease, it may not be considered a medical device. On the other hand, if the AI does the same task but looks for patients similar to the patient at hand, it is highly likely that this software would be classified as a medical device.
What now?
After reading this article, you should have a better understanding of when your AI product might be considered a medical device. If you are still unsure, please feel free to reach out to us.
If you have determined that your AI product is not a medical device: Congratulations! In some cases, our clients need assistance in formulating solid arguments to explain why their product is not regulated in order to reduce the regulatory risk for various stakeholders. If you need assistance with this, please do not hesitate to contact us.
If your AI product is a medical device, you may benefit from our upcoming article, MDR classification of AI software, which will help you to determine the class of your AI product. We hope that this article will provide you with the resources you need to ensure the success of your AI product.
TL;DR:
If your AI software has anything to do with the diagnosis, prevention or monitoring of a disease, it is almost certainly a medical device.
Possible Exceptions:
The software is only processing information in a trivial way, e.g. storage, archival, embellishment, simple search. This is highly unlikely when AI is involved.
The AI is not for the benefit of individual patients e.g. AI for the analysis of population data or aggregation of scientific literature. With current progress in NLP, this might be increasingly relevant in the future.


